Targeting acute kidney injury with LSALT peptide
LSALT Peptide (Metabloktm)
LSALT peptide is the company’s lead drug candidate for the prevention of Acute Kidney Injury (AKI) and other common inflammation injuries in the kidneys, liver and lungs.
Arch is focused on a path to drug approval for LSALT peptide to prevent AKI and organ inflammation in common injury and disease applications where there are no effective drugs or treatments available in the market today.

Click to WATCH LSALT peptide At work
Video data behind Arch lead scientists publication in Cell shows the real-time effects of LSALT peptide when used to prevent inflammation in pre-clinical models of AKI, similar to the inflammation found in CS-AKI – click to watch the videos below.
The company is sponsoring an ongoing Phase II international multi-center, randomized, double-blind, placebo-controlled study of LSALT peptide targeting cardiac surgery-associated acute kidney injury (CS-AKI). Drug approval for treating this type of inflammation related kidney injury may enable off-label use for other indications such as lung or liver inflammation injury, other AKI indications and septic shock.
A NEW PEPTIDE DRUG CANDIDATE AND DPEP1 INHIBITOR
Arch believes that LSALT peptide has the potential to deliver a major breakthrough in the treatment of common injuries and diseases where organ inflammation affects millions of patients globally every year.
In August 2019, a scientific team led by Arch scientists Dr. Donna Senger and Dr. Stephen Robbins published a paper in the journal Cell describing a novel mechanism of action for organ inflammation.
In the paper, the enzyme dipeptidase-1 (DPEP1) was identified as a major neutrophil (white blood cell) adhesion receptor on the lung, liver and kidney endothelium – additionally, DPEP1 was shown to be the target of LSALT peptide – identifying a target for neutrophil-driven inflammatory diseases of the lungs, differing from typical anti-inflammatory drugs by targeting this novel adhesion receptor rather than targeting individual cytokines.
In 2024 Arch scientists were published in the journal BMJ Open, this time with the Proof of Concept study results for the company’s 2021-2022 Phase II trial. The data from that trial provided the first-ever evidence validating Dipeptidase-1 (DPEP1) as a mediator of organ inflammation and therapeutic target in humans. In addition, the article included crucial evidence that LSALT peptide was well tolerated with no safety issues related to the drug.
BMJ Open – March 2024
Publication of proof of concept study of LSALT peptide
Phase II trial results provided first-ever evidence validating Dipeptidase-1 (DPEP1) as a mediator of organ inflammation and therapeutic target in humans.
Arch is developing LSALT peptide to treat acute kidney injury (AKI) and organ damage caused by inflammation
The current LSALT peptide Phase II cardiac surgery-associated acute kidney injury (CS-AKI) trial began dosing patients in March 2024. The trial began with five active hospital sites in Turkey, and has since added three hospital sites in Canada (one pending activation). The complete study is planned to include up to 240 patients in Turkey, Canada and the United States.
- August 2025, announcement of first patient successfully dosed at Toronto General Hospital, Canada’s largest academic health sciences centre and a global leader in cardiac care and research.
- November 2024, announcement of dosing of the first patient dosed at the University of Calgary, Cumming School of Medicine, the first Canadian site to join and activate in the trial.
- April 2024, announcement of St Michael’s Hospital and Toronto General Hospital (UHN) joining the trial.
- March 2024, dosing of first patient in Turkey marking the commencement of the Phase II CS-AKI trial.
- March 2024, announcement of first Canadian site to join the trial, beginning with the University of Calgary, Cumming School of Medicine.
- March 2024, publication of previous Phase II trial results providing further scientific rationale to advance LSALT peptide to prevent leukocyte recruitment and organ inflammation for other indications.
- June2023, successfully obtained the U.S. FDA’s permission to proceed with the Phase II trial to prevent CS-AKI.
LSALT PEPTIDE CLINICAL TRIALS
The Phase II trial to target cardiac surgery associated-acute kidney injury (CS-AKI), began enrolling subjects in March 2024.
The commencement of the Phase II trial followed the U.S. FDA’s review of the Company’s investigational new drug (IND) application submitted to the FDA’s Division of Cardiology and Nephrology in May, 2023. The IND application included preclinical data, Phase I and Phase II clinical data to date, manufacturing processes, and the protocol design for the CS-AKI Phase II trial.
CS-AKI is often caused by ischemia-reperfusion injury (IRI) that reduces blood flow (ischemia) and thus oxygen in the kidney causing kidney cell damage. Once blood flow is restored to normal (reperfusion), inflammation is triggered and injury to kidney cells is exacerbated. In the worst cases of AKI, kidneys fail, leading to kidney dialysis or kidney transplant. At present, there are no therapeutic treatments available to prevent or treat CS-AKI or IRI.
The CS-AKI trial is planned to recruit up to 240 patients and is an international, multi-site, double-blind, placebo-controlled study to be conducted in hospital sites in Turkey, Canada and the US.
Patient enrollment and dosing began in March of 2024, following approvals from local health authorities, ethics committees and internal review boards. There is an independent Data Safety Monitoring Board which is monitoring the safety of the patients enrolled in the trial.
EXPANDED DOSING AND SAFETY
In April 2023, the company announced that it had safely increased the maximum daily dose of LSALT peptide to 20 mg per day in healthy, normal volunteers in a phase II human safety trial in Australia. Prior to this dose escalation trial, LSALT peptide was administered at a maximum of 5mg once daily, for up to 14 days, in hospitalized patients during a Phase II trial targeting inflammation in the lungs.
About the 2020-21 Phase II ARDS and AKI Trial
Upon completion of a Phase I trial in 2020, Arch also completed a Phase II trial conducted in the United States, Turkey and Canada. The Phase II international, multi-center, randomized, double-blind, placebo-controlled, proof of concept trial was designed to investigate LSALT peptide’s efficacy in the prevention of acute inflammation in the kidney, known to trigger acute respiratory distress syndrome (ARDS) and acute kidney injury (AKI) in hospitalized patients infected with SARS-CoV-2
Additional secondary endpoints included reducing coagulopathy, cardiomyopathy and acute liver injury experienced by hospitalized SARS-CoV-2 patients.
In 2024 in a peer reviewed publication in the journal BMJ Open, the Arch clinical team published results from the previous Phase II findings which support dipeptidase-1 (DPEP-1) as a relevant therapeutic target for diseases of the lung, kidneys and liver where inflammation plays a major role. For the first time in this publication, the team also disclosed new biomarker data from the SARS-CoV-2 Phase II trial which provided further scientific rationale for Arch to bring LSALT peptide into larger trials to inhibit DPEP-1 mediated organ inflammation.
The ARDS and AKI trial dosed 61 patients in an international, multi-center, randomized, double-blind, placebo-controlled, proof of concept study of LSALT peptide as prevention of organ inflammation known to trigger acute respiratory distress syndrome (ARDS) and acute kidney injury (AKI).
Additional information about the company’s Phase II trials can be found at ClinicalTrials.gov:
- 2023 CS-AKI Phase II
https://clinicaltrials.gov/study/NCT05879432 - 2020-2021 ARDS and AKI Phase II
https://www.clinicaltrials.gov/study/NCT04402957
This Phase III study was an adaptive, randomized, open-label, controlled clinical trial, in collaboration with countries around the world through the World Health Organization.
In the later stages of the global pandemic, subjects were randomized, across one, two, three or up to four separate randomizations (including LSALT Peptide vs. Standard of care, alongside other therapies), to receive either standard-of-care products or the study medication plus standard of care, while being hospitalized for COVID-19.
The primary outcome measures for LSALT Peptide measured patients’ number of respiratory support free days.
The secondary outcome measures included measures of clinical effectiveness, need for invasive mechanical ventilation, CU admission, hospital and ICU length of stay, days alive and free of vasopressors, ventilation, and renal replacement therapy (RRT), severe adverse events, and 1, 3 and 6 months mortality, laboratory (cardiac pulmonary coagulation, and renal) and clinical evaluation using standardized pre-defined COVID-19 virtual and/or in person follow-up and mortality after randomization
Due to waning numbers of hospitalizations at the end of the pandemic, there were not enough patients recruited into the CATCO trial to make any conclusions on trial data related to LSALT peptide dosing. Arch has since refocused on further testing LSALT peptide in other indications where organ inflammation and injury is a problem.
The company has successfully conducted multiple Phase I human trials of LSALT peptide. The most recent, completed in 2023, was a double-blind, placebo-controlled and randomized dose-escalation study, where single and multiple ascending doses were administered to evaluate the safety and pharmacokinetic profile of LSALT peptide.
About the 2023 Phase I Trial
Six participants in each group of the trial received a daily dosing of LSALT peptide for one day followed by three consecutive days of dosing. Two people per group received a placebo with the same dosing schedule. In the first group, six people received a once daily, 10 mg intravenous (IV) dose of LSALT peptide. In the second group, six volunteers received a 10 mg IV dose of LSALT peptide twice a day, approximately every 12 hours. In both groups, LSALT peptide met the primary endpoints of safety and tolerability.
In April 2023, the company announced that it had safely increased the maximum daily dose of LSALT peptide to 20 mg per day in healthy, normal volunteers in a phase II human safety trial in Australia. “Prior to this dose escalation trial, LSALT peptide was administered at a maximum of 5mg once daily, for up to 14 days, in hospitalized patients during a Phase II trial targeting inflammation in the lungs.” (Arch Biopartners April 2023)
EARLIER LSALT peptide Phase I Trials
In March 2020, Arch announced that LSALT peptide had successfully met the primary endpoints of safety and tolerability in a Phase I Human Trial involving 52 healthy, normal volunteers. The placebo-controlled, double-blind study was conducted in Australia.
Previously, in December 2019, Arch made an announcement that LSALT peptide had achieved primary endpoints of safety and tolerability. Based on the initial completion of the Phase I trial, Arch announced that it had expanded the dosing in trial, to increase the dose range for future Phase II studies.
PreClinical Data and Background
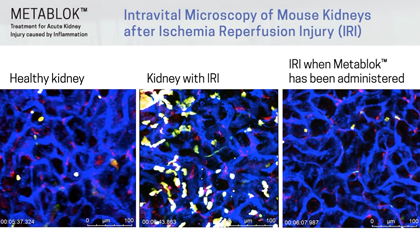
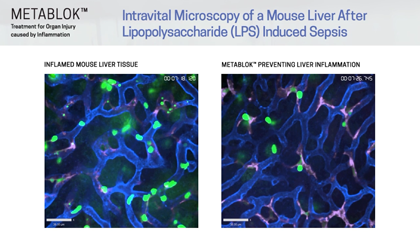
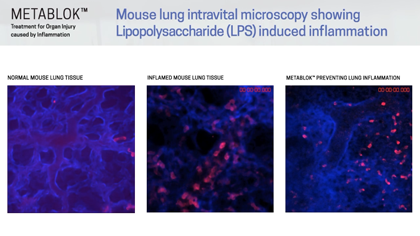
Arch scientists have demonstrated LSALT peptides ability to block the inflammatory response triggered in the kidney with ischemia/reperfusion (IRI) induced injury and in the lung and liver with lipopolysaccharide (LPS) induced inflammation. Currently, there are no specific or effective treatments to prevent acute kidney injury or organ injury caused by inflammation more broadly.
Following publication in the peer-reviewed journal Cell in August 2019, Arch Scientists released a series of preclinical videos using Intravital Microscopy data to show the real-time effects of LSALT peptide when used to reduce inflammation in preclinical mouse models.
The video series includes demonstrations of LSALT peptide compared to normal and injured organs. In each video, the treated kidneys, liver and lungs are shown to have a reduction of the action of inflammation present in the organ (see ‘Videos & Media, at left).
From the publication in Cell, August 2019
“Using biochemical, genetic, and confocal intravital imaging approaches, we identified dipeptidase-1 (DPEP1) as the target and established its role as a physical adhesion receptor for neutrophil sequestration independent of its enzymatic activity. Importantly, genetic ablation or functional peptide blocking of DPEP1 significantly reduced neutrophil recruitment to the lungs and liver and provided improved survival in models of endotoxemia.”
Inflammation Based Disease
Inflammation is a localized physical condition that involves the activation of the immune system in response to infection, tissue injury, or autoimmunity. Inflammation is involved in the pathogenesis of many diseases and contributes to organ dysfunction and failure.
LSALT peptide was originally investigated as a new method to prevent organ inflammation related to sepsis.
Sepsis represents a large unmet medical need in the world today. Sepsis alone occurs in 1 to 2% of all hospitalizations in the US. It affects at least 700,000 individuals per year.
Permanent organ damage can occur in patients who survive sepsis. Under the current standard of care, mortality rates are over 20% for sepsis and over 50% for septic shock.
Intellectual Property
LSALT peptide (Metablok) was invented by Arch scientists Dr. Stephen Robbins, Dr. Donna Senger, Dr. Jennifer Rahn and their University of Calgary colleague, Dr. Paul Kubes. The inventors have assigned the intellectual property to the Company.